[ad_1]
Fool the new coronavirus once and it can’t cause cell infection, new research suggests.
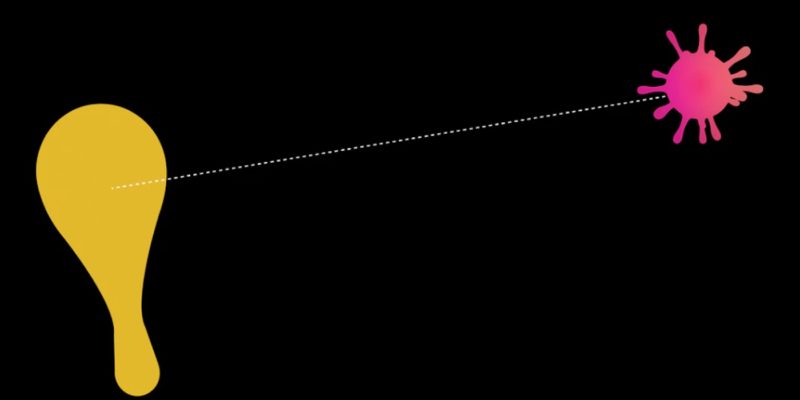
Scientists have developed protein fragments, called peptides, that fit neatly into a groove in the SARS-CoV-2 spike protein that it would normally use to access a host cell. These peptides effectively trick the virus into “shaking hands” with a replica rather than the actual protein on the surface of a cell that lets the virus in.
Previous research had determined that the new coronavirus binds to a receptor protein on the surface of a target cell called ACE2. This receptor is found on certain types of human cells in the lung and nasal cavity, providing SARS-CoV-2 with many access points to infect the body.
For this work, scientists at Ohio State University designed and tested peptides that resemble ACE2 enough to convince the coronavirus to bind to them, an action that blocks the virus’s ability to actually enter the cell.
“Our goal is that every time SARS-CoV-2 comes into contact with peptides, the virus is inactivated. This is because the Spike virus protein is already attached to something that it needs to use to bind to the cell, ”said Amit Sharma, co-lead author of the study and assistant professor of veterinary biosciences at Ohio State. “To do this, we have to get to the virus while it is still outside the cell.”
RELATED: 2 New Nasal Sprays That Kill COVID-19 Look Remarkably Effective
The Ohio State team plans to deliver these manufactured peptides in a nasal spray or aerosol surface disinfectant, among other applications, to block circulating access points of SARS-CoV-2 with an agent that prevents its entry into target cells.
“With the results that we generate with these peptides, we are well positioned to move into the product development steps,” said Ross Larue, co-lead author and research assistant professor of pharmacy and pharmacology at Ohio State.
The study appears in the January issue of the journal Bioconjugate chemistry.
SARS-CoV-2, like all other viruses, requires access to living cells to do its damage: Viruses hijack cellular functions to make copies of themselves and cause infections. The very rapid replication of the virus can overwhelm the host system before immune cells can muster an effective defense.
POPULAR: 10 Positive COVID Updates From Around The World – 2021 Is Looking Brighter
One of the reasons this coronavirus is so infectious is because it binds so tightly to the ACE2 receptor, which is abundant in the cells of humans and some other species. The spike protein on the surface of SARS-CoV-2, which has become its most recognizable feature, is also critical to its successful binding to ACE2.
Recent advances in protein crystallization and microscopy have made it possible to create computer images of specific protein structures alone or in combination, such as when they are attached to each other.
Sharma and his colleagues closely examined the images of the Pico SARS-CoV-2 and ACE2 protein, focusing on how their interactions occur and what connections are required for the two proteins to lock into place. They noticed a spiral ribbon-like tail in ACE2 as the focal point of the accessory, which became the starting point for designing peptides.
CHECK OUT: She was demoted, doubted and rejected, but now her work is the basis of the Covid-19 vaccine
“Most of the peptides we design are based on the tape that comes into contact with the spike,” said Sharma, who also holds a position on the faculty in microbial infections and immunity. “We focus on creating the shortest possible peptides with the minimum essential contacts.”
The team tested various peptides as “competitive inhibitors” that could not only bind safely to the spike proteins of SARS-CoV-2, but also prevent or slow viral replication in cell culture. Two peptides, one with minimal contact points and one larger, were effective in reducing SARS-CoV-2 infection in cell studies compared to controls.
Sharma described these findings as the beginning of a product development process that will be continued by the team of virologists and pharmaceutical chemists collaborating on this work.
LOOK: We use 6 billion masks a day, but scientists have a cool way of turning them into roads
“We are taking a multi-pronged approach,” Sharma said. “With these peptides, we have identified the minimum contacts necessary to inactivate the virus. In the future, we plan to focus on developing aspects of this technology for therapeutic purposes.
“The goal is to effectively and potently neutralize the virus, and now, due to the emergence of variants, we are interested in testing our technology against emerging mutations.”
(Source: State of ohio – Photo by @visuals)
SHARE the virus-free handshake with friends on social media …
[ad_2]
Original source