[ad_1]
Long-lasting fast-charging batteries are essential to the expansion of the electric vehicle market, but today’s lithium-ion batteries fall short of what is needed: they are too heavy, too expensive and take too long to charge.
For decades, researchers have tried to harness the potential of solid-state lithium metal batteries, which retain substantially more energy in the same volume and charge in a fraction of the time compared to traditional lithium-ion batteries.
“A lithium metal battery is considered the holy grail of battery chemistry due to its high capacity and energy density.” said Xin Li, Associate Professor of Materials Science at Harvard’s John A. Paulson School of Engineering and Applied Sciences (SEAS). “But the stability of these batteries has always been poor.”
Now, Li and his team have designed a stable lithium metal solid-state battery that can be charged and discharged at least 10,000 times – many more cycles than previously demonstrated – at high current density. The researchers paired the new design with a commercial, high-energy-density cathode material.
This battery technology could extend the lifespan of electric vehicles to gasoline cars by 10-15 years without the need to replace the battery. With its high current density, the battery could pave the way for electric vehicles that can be fully charged in 10 to 20 minutes.
“Our research shows that the solid-state battery could be fundamentally different from the commercial liquid-electrolyte lithium-ion battery,” Li said. “By studying its fundamental thermodynamics, we can unlock superior performance and seize its many opportunities.”
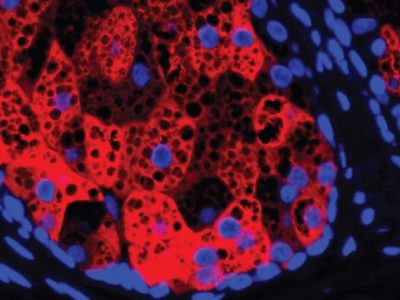
The big challenge with lithium metal batteries has always been chemistry. Lithium batteries move lithium ions from the cathode to the anode during charging. When the anode is made of lithium metal, needle-like structures called dendrites form on the surface. These structures grow as roots in the electrolyte and pierce the barrier that separates the anode and cathode, causing the battery to short-circuit or even catch fire.
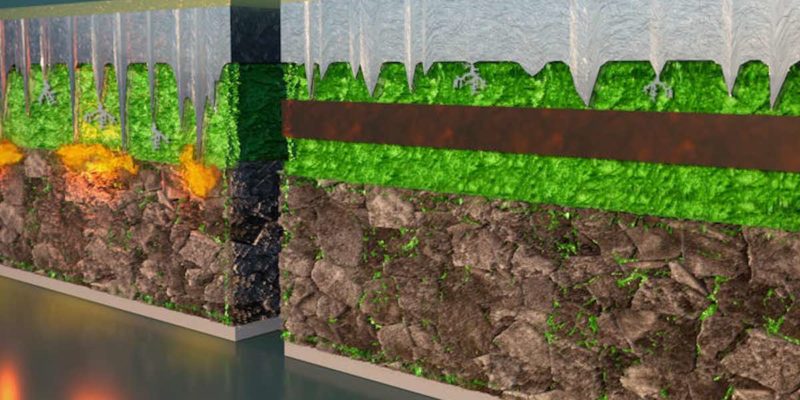
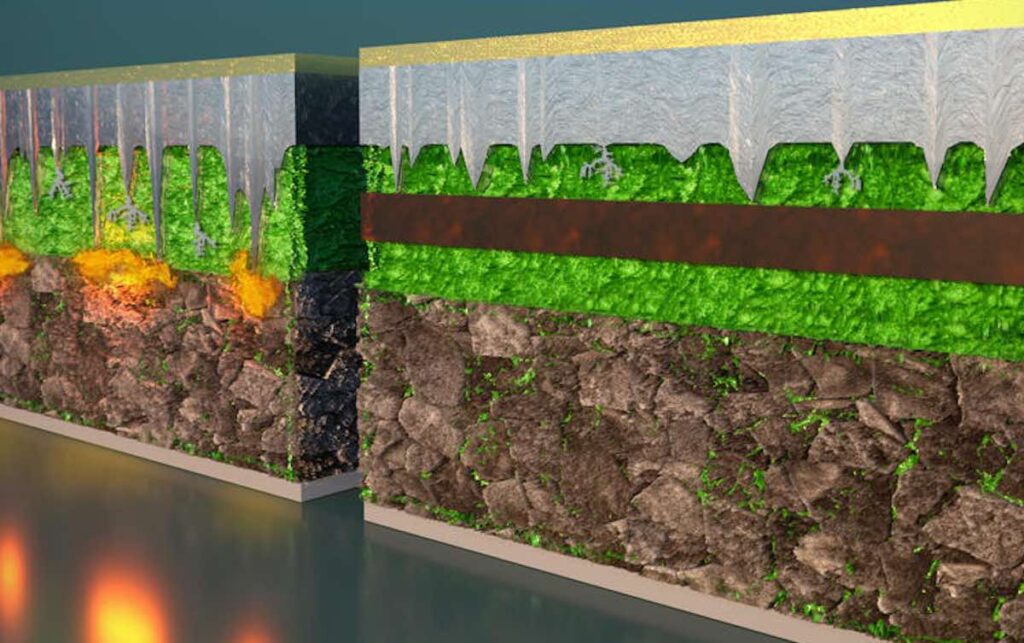
To overcome this challenge, Li and his team designed a multilayer battery that pairs different materials of different stabilities between the anode and the cathode. This multilayer, multimaterial battery prevents the penetration of lithium dendrites by not stopping them completely, but by controlling and containing.
Like a BLT sandwich
Think of drums as a BLT sandwich. First comes the bread, the lithium metal anode, followed by the lettuce, a layer of graphite. Next, a layer of tomatoes, the first electrolyte, and a layer of bacon, the second electrolyte. Finish off with another layer of tomatoes and the last piece of bread – the cathode.
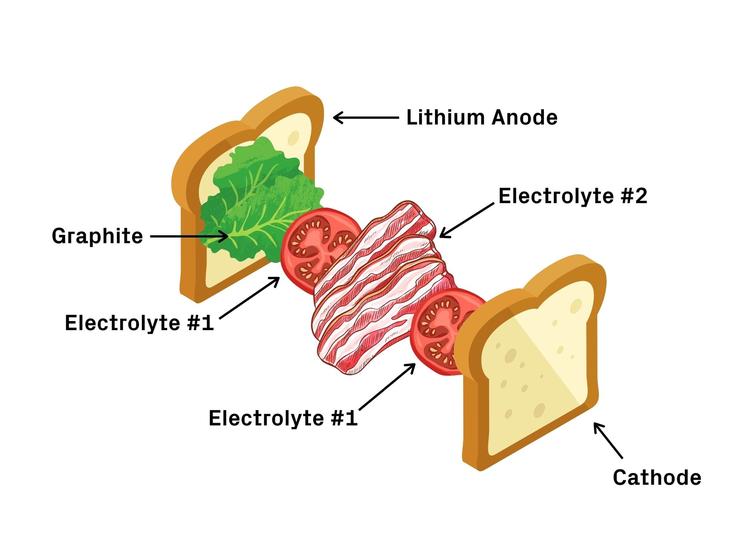
The first electrolyte (chemical name Li5.5PS4.5Cl1.5 or LPSCI) is more stable with lithium but prone to dendrite penetration. The second electrolyte, (Li10Ge1P2S12 or LGPS) is less stable with lithium but appears immune to dendrites. In this design, the dendrites are allowed to grow through the graphite and the first electrolyte, but stop when they reach the second. In other words, dendrites grow through lettuce and tomato, but stop at bacon. The bacon barrier prevents dendrites from passing through and shorting the battery.
“Our strategy of incorporating instability to stabilize the battery feels counterintuitive, but just as an anchor can guide and control a screw crashing into a wall, our multi-layer design guide can also control dendrite growth.” said Luhan Ye, a co-author of the paper and a graduate student at SEAS.
“The difference is that our anchor quickly becomes too tight for the dendrite to pierce, so dendrite growth stops,” Li added.
The battery also repairs itself automatically; its chemistry allows it to fill in the holes created by dendrites.
“This proof-of-concept design shows that lithium metal solid-state batteries could be competitive with commercial lithium-ion batteries,” Li said. “And the flexibility and versatility of our multilayer design makes it potentially compatible with mass production procedures in the battery industry. Scaling it up to the commercial battery won’t be easy and there are still some practical challenges, but we think they will be overcome. “
The investigation was published in Nature, and supported by Dean’s Competitive Fund for Promising Scholarship at Harvard University and the Harvard Data Science Initiative Competitive Research Fund.
BOOST this development to your technical friends on social media …
[ad_2]
Original